Bioluminescence
Bioluminescence is light produced by a living organism: bacteria, fungi, crustaceans, fish, insects, molluscs, millipedes and centipedes. While it is fascinating to see bioluminescent animals in nature, the chemical basis of bioluminescence has proved fruitful in producing applications in areas as diverse as cancer research and forensic sciences.
Bioluminescence is sometimes confused with other phenomena such as fluorescence and luminescence. Fluorescence is the production of light at one wavelength in response to illumination at another wavelength. In an application you might be familiar with, some products fluoresce white under “black light” (ultraviolet) illumination. Scorpions are noted for the fact that their cuticle fluoresces cyan-green under ultraviolet light. Scorpion collectors use this at night as they hunt scorpions using a hand-held ultraviolet lamp. Some researchers have indicated that scorpion autofluorescence has some biological function, for example, in extra-ocular photoreception, but it remains possible that it is purely a by-product of the protein composition of the cuticle.
|
What insects are capable of bioluminescence?
Coleoptera and Diptera are the only orders that have well-documented bioluminescent members. A paper published in 2012 argued that a group of cockroaches is bioluminescent. The report of their bioluminescence relies on one observation by a field collector made in 1977. The insects are rare and are found in the Amazon region. When they are kept in captivity they do not bioluminesce so the original observation remains doubtful. Sometimes bioluminescent fungi can inhabit the bodies of dead or dying insects so it is possible that this explains the one and only observation of bioluminescence in cockroaches.
Coleoptera
- Fireflies: Lampyridae (fireflies and glow-worms),
- Phengodidae (railroad worms)
- Click Beetles Elateridae (click beetles)
Diptera: Keroplatidae
- Genus Arachnocampa: “glowworms” found in Australia and New Zealand.
- Genus Orfelia: “dismalites” from North America
Origins of beetle bioluminescence
It is widely accepted that bioluminescence originated in larval beetles where it had, and still has, an aposematic function because (1) firefly larvae bioluminesce more brightly when they are disturbed and (2) light organs are present in larvae of some species where the adults do not bioluminesce. In larvae, it appears that the light-producing cells are derived from fat body. Behavioural experiments with toads show that they learn to avoid bioluminescent larvae after feeding upon them; they associate bioluminescence with distaste.
Larval and adult light organs are unrelated, although adult light organs also appear to be evolutionarily derived from fat body. It appears that the larval “steady glowing” system has been co-opted into a different tissue by adults and they subsequently evolved neural regulation capabilities to produce the complex flash signals seen in some species. Just like any mating system, bioluminescence usage is diverse across the Lympyridae. Also, it is sometimes intertwined with use of pheromones.
Phylogenetic studies that match the use of pheromones and light-based signals against evolutionary relatedness suggest that sexual signalling in Lampyridae evolved from using pheromones alone (probably a diurnal mating system), to a combination of pheromones and light signals (probably a dusk mating system), to the use of bioluminescence signalling alone. The change from pheromone-based to light-based mating systems occurred alongside a switch in the “temporal niche” used for mating.
In the most complex cases of bioluminescence usage, the males and females have their own specific flash characteristics, allowing the males to orientate toward the females. Commonly, females are apterous and larviform; they cannot readily orient to males. Female fireflies show varied degrees of neoteny, i.e. retention of juvenile traits. At one extreme, females and males are similar, whereas at the other extreme, females are grub-like and resemble wingless, immature stages.
In some species, found in south-eastern Asia, males aggregate in mangrove trees where they flash in unison, producing synchronised bioluminescence flashes.
Beetle light organ structure
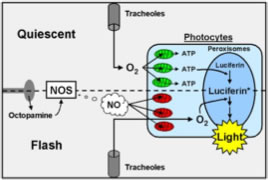
The light organ of adult beetles is located ventrally in the posterior segments. The cuticle is transparent. Underneath the cuticle an array of symmetrically packed modified fat body cells are the light-emitting cells (the photocytes). They are closely associated with many trachea that provide respiratory capacity and also act as a light reflector. They are innervated by neurons that release the biogenic amine, octopamine.
The photocytes are densely packed with peroxisomes; intracellular bodies where the light reaction takes place. The regulation of flashing is not completely understood; however, a numbe of experimental approaches have revealed the neural and cellular basis of flash regulation. There are two main theories, both based on restricting and allowing oxygen access to the photocytes after a nerve impulse is received at the light organ.
Nitrous oxide-based mechanism
In the diagram, on the top half, oxygen from trachea is consumed by respiration in the photocyte mitochondria (green), consequently insufficient oxygen reaches the peroxisomes to initiate a flash. After a nerve impulse (bottom half), octopamine is released which transiently activates nitrous oxide. The nitrous oxide diffuses toward the mitochondria where it inhibits oxygen use by photocyte mitochondria (red). Oxygen diffuses through the photocytes to the peroxisomes where it triggers the light-producing reaction. The reaction is based upon ATP-based activation of the substrate molecule, luciferin, by luciferase.
Tracheal fluid mechanism 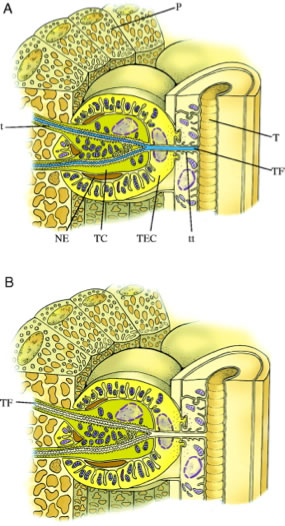
The trachea fill with fluid when light is not emitted. Neural stimulation leads transient increase in the osmotic potential of the tracheolar cell leading to decreased tracheolar fluid levels. The decreased diffusional barrier allows more oxygen supply to the photocytes, relieving intracellular anoxia and enabling light emission.
As tracheolar cell osmotic potential returns to the resting state, tracheolar fluid levels increase, oxygen diffusion to the photocytes is decreased, intracellular anoxia occurs in the photocytes and light emission is inhibited.
The glowworm fly, genus Arachnocampa
The glowworms of Australia and New Zealand are the larval stages of a fly, genus Arachnocampa, Family Keroplatidae. Eight species are found in Australia and one in New Zealand. All produce light from a posteriorly-located light organ composed of the terminal cells of the malpighian tubules, closely apposed to a tracheal reflector. The function of the bioluminescence is to attract prey.
Unique to glow-worms, light output is bright and persistent throughout the night. Bioluminescence in glow-worms is produced by a reaction involving the protein luciferin, the enzyme luciferase and ATP in the presence of oxygen. The light is blue-green in colour (peak at 485 nm). Given the ATP requirement and the many mitochondria in the light-producing cells, bioluminescence could impose an appreciable energetic cost but measurements of metabolic cost to the whole animal indicated it used less energy than did movement within the snare. This is because the reaction is efficient in that it produces light but no heat; however the light-producing cells might still be using a lot of energy.
Regulation of the level of bioluminescence appears to be under neural control. Exposure to anaesthetics such as ether and CO2 causes an acute release of light, even if larvae are not glowing at the time of exposure, suggesting that the default state is for the light organ to be active, and light output is inhibited by detection of daylight and severe disturbances. Ligation of larvae behind the head or removal of the head causes larvae to glow dimly for a long period, suggesting the inhibitory signal comes from the brain. The effect of the anaesthetic, carbon dioxide, on the isolated light organ indicates that the inhibitory effect is at least partially mediated at the light organ itself. Blocking of neural signals from the CNS through ligation leads to uncontrolled release of bioluminescence but light is emitted at relatively low levels compared to under anaesthesia. Candidate biogenic amines were introduced by several methods: feeding prey items injected with test solution, injecting the whole larva, injecting a ligated section containing the light organ or bathing the isolated light organ in test solution. Using these methods, dopamine, serotonin and tyramine do not affect bioluminescence output. Exposure to elevated octopamine via feeding, injection or bathing of the isolated light organ indicates that it is involved in the regulation of repression.
Administration of the octopamine antagonists, phentolamine or mianserin, results in very high bioluminescence output levels, similar to the effect of anaesthetics, but only mianserin acts directly on the light organ.
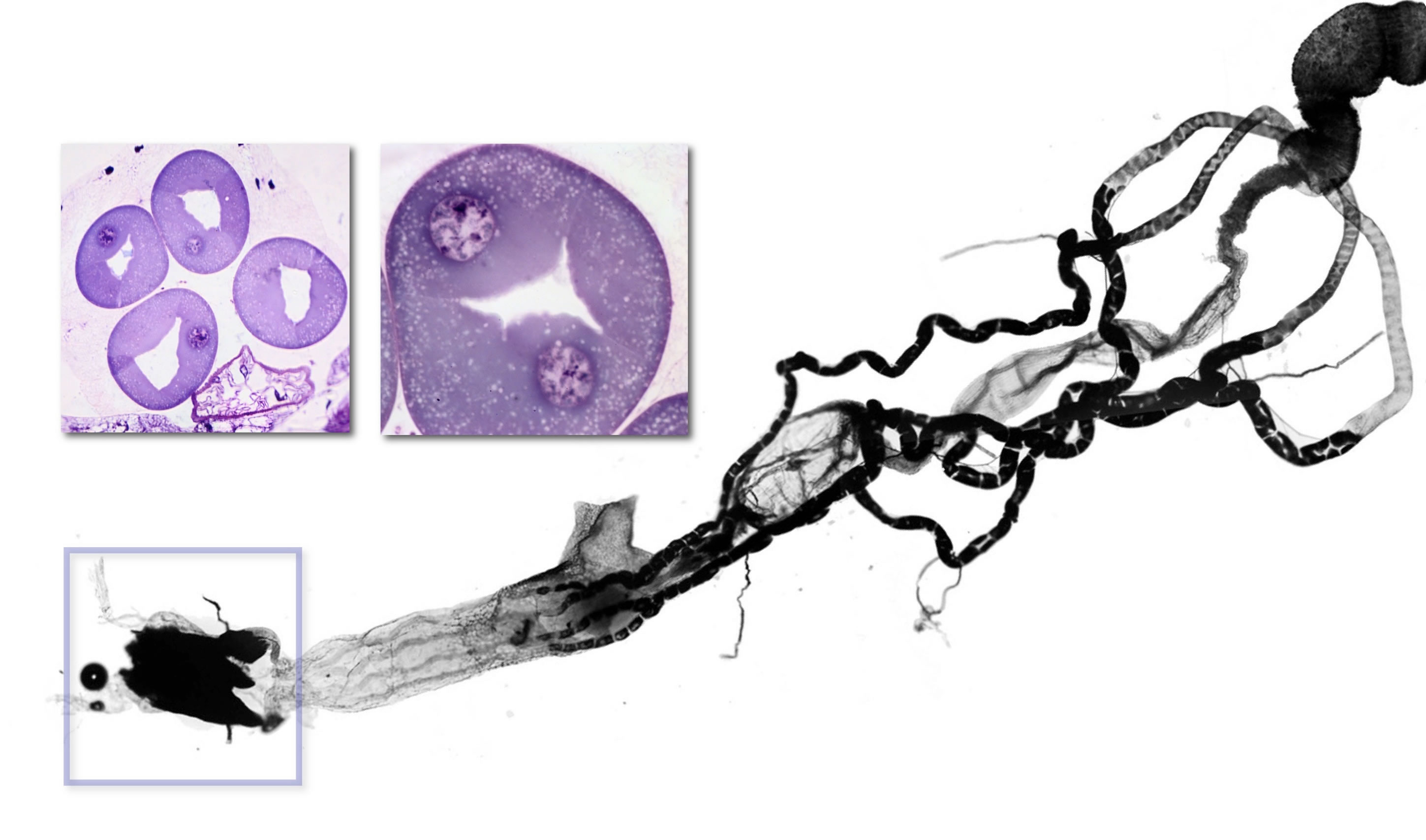
The light organ of Arachnocampa. The gut and malpighian tubules have been dissected from a larva. The four malpighian tubules originate at the midgut-hindgut junction. They initially lie free in the haemocoel, then become closely applied to the surface of the hindgut. The light organ (box) appears dark because the tracheal reflector does not transmit light in this micrograph. The sections show the large cells of the light organ surrounding a central lumen.
A number of sensory inputs have been shown to result in the up- or down-regulation of light output. Vibration induced by tapping their chamber caused larvae to rapidly increase their light output, followed by a return to undisturbed levels over about 10 min. The fact that vibrational disturbances cause increased output when larvae are already glowing, suggests that energy or metabolites are held in reserve during the normal glowing cycle.
In New Zealand, tourism takes place in several caves that feature large glow-worm populations. Tours range from walk-through tours, to boat trips, to adventure-style blackwater rafting in which clients float through glow-worm chambers on inflated inner tubes. Tour operators have noticed that sudden, sharp sounds can cause apparent increases in glow-worm light intensity and this has been confirmed photographically (Merritt, unpublished data).
The function of the light increase remains obscure: perhaps vibration causes resting insects, potential prey items, to take flight. A simultaneous increase in glow-worms' light output might increase the chances a prey item, disturbed by the same stimulus, will be attracted and caught. Other possibilities are that it is an aposematic response or that it is simply a startle reaction.
Exposure to light causes actively bioluminescing glow-worms to douse their light within a few minutes and recover within 30 to 60 minutes.
References
Branham MA, Wenzel JW. 2001. The evolution of bioluminescence in cantharoids (Coleoptera: Elateroidea). Fla Entomol:565-586.
Rigby LM, Merritt DJ. 2011. Roles of biogenic amines in regulating bioluminescence in the Australian glowworm Arachnocampa flava. J Exp Biol 214:3286-3293.
Willis R, White C, Merritt D. 2011. Using light as a lure is an efficient predatory strategy in Arachnocampa flava; an Australian glowworm. J Comp Physiol (B) 181:477-486.
TOPIC REVIEWDo you know…?
|
End of the Module: Bioluminescence
For more information about Glowworms and research, see:
Dave Merritt's Glowworm website
 Go on to the next Module:Thermoregulation
Go on to the next Module:Thermoregulation


 Minilecture:
Minilecture: