Dormancy: Diapause and Quiescence
Objectives 
- Understand the difference between diapause and quiescence
- Know the common cues that initiate insect diapause, and how they are detected
Topic Outline
- Overview, cues and controls of diapause
- Diapause in different stages of development
- genetics of diapause
Activities
- there are no minlectures for this module yet
Overview
Dormancy is suppressed development in response to adverse environmental conditions. There are two different types of dormancy: quiescence and diapause.
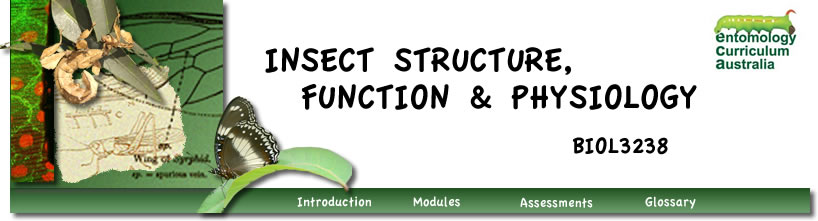
Quiescence is “an immediate direct response to a limiting factor, such as cessation of development if temperatures fall below a threshold but immediate resumption of development if temperatures rise above it.” This is sometimes called torpor.
 There is a special form of dormancy, termed diapause, in which the insect’s physiology is “switched” into an alternative state, adapting the insect to impending hardship. Diapause is “a more profound interruption that routes the metabolic program of the organism away from direct developmental pathways into a much more clearly organised break in development that is not controlled simply by the direct action of environmental factors and which in nature precedes the advent of adverse conditions” (from Danks, 1987).
There is a special form of dormancy, termed diapause, in which the insect’s physiology is “switched” into an alternative state, adapting the insect to impending hardship. Diapause is “a more profound interruption that routes the metabolic program of the organism away from direct developmental pathways into a much more clearly organised break in development that is not controlled simply by the direct action of environmental factors and which in nature precedes the advent of adverse conditions” (from Danks, 1987).
 Diapause represents a collection of behavioural and physiological processes that enhance the insect’s survival. Evidence suggests that diapause is initiated by one or a few master genes. This triggers a cascade of other genes that alter the condition of the entire organism. Lipid and protein contents of the haemolymph increase. Sometimes glycerol accumulates in the haemolymph for freeze-tolerance, and oxygen use drastically declines to about 10% of the non-diapause state, along with heartbeat rate and metabolic rate in general. Not surprisingly, the hormonal system is most closely involved in the regulation of diapause because it involves long-term physiological and behavioural changes.
Diapause represents a collection of behavioural and physiological processes that enhance the insect’s survival. Evidence suggests that diapause is initiated by one or a few master genes. This triggers a cascade of other genes that alter the condition of the entire organism. Lipid and protein contents of the haemolymph increase. Sometimes glycerol accumulates in the haemolymph for freeze-tolerance, and oxygen use drastically declines to about 10% of the non-diapause state, along with heartbeat rate and metabolic rate in general. Not surprisingly, the hormonal system is most closely involved in the regulation of diapause because it involves long-term physiological and behavioural changes.
The term “aestivation” is commonly used for diapause in response to high temperatures in the tropics, and “overwintering” for diapause in response to winter conditions in temperate zones.
Some insects undergo obligate diapause which requires the diapause stage to be completed before development can ensue. Most often, these species are univoltine (one generation per year) and the egg is frequently the diapausing (overwintering) stage.
Others undergo facultative diapause which is not obligatory: it is undertaken only if appropriate conditions are met. Frequently, these are bivoltine or multivoltine.
Diapause can occur at any stage of the life cycle, but is most common in the stages that are relatively quiescent such as the embryo and the pupa. The diapausing stage is species specific, i.e. one species will diapause at the same stage(s). There are a few rare examples of species which can diapause at several stages of their life cycle.
The stimuli that initiate diapause may be token stimuli, i.e. the stimuli which initiate diapause do not cause adversity, rather they are signals of impending adversity. Day length is a very common stimulus for the onset of diapause.
Some authors suggest that the term “hibernation” should only be applied to mammals, which are warm-blooded.
Cues
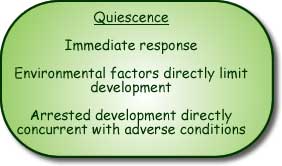
Left: Critical photoperiod for diapause induction in populations of a long-day species, Sarcophoga bullata, from Illinois and Missouri (From Denlinger, D., 1972.)
Right: Critical photoperiod for diapause induction in two short-day species (1) Stenocranus minutus at 20° and (2) Bombyx mori, at 15°. (From Danilevskii, A., 1965.)
 Photoperiod a common cue. It is closely correlated with seasonal change. See Chapman section 15.6
Photoperiod a common cue. It is closely correlated with seasonal change. See Chapman section 15.6
Transition is abrupt and based on precise time-keeeping mechanism.
Most diapausing insects show a “long-day” response to photoperiod. They are active in summer and diapause over winter. Short day lengths induce diapause. Sarcophagids are an example of a long-day species (see graph). Transition point (critical day length) for diapause induction is very precise.
Compare with graph for short day species, such as the silkworm, Bombyx mori.
The photosensitive period is usually well in advance of the diapause stage. For example, Sarcophaga (fleshfly) embryonic or larval exposure to short day lengths is sufficient to induce diapause in the pupa (Diagram).
Temperature is another common cue.
Quantity and quality of food
- food deprivation in mosquito larvae
- food quality decreases as plants senesce, indicator of impending dry season
- water content of food in fleshflies
Sequence of requirements
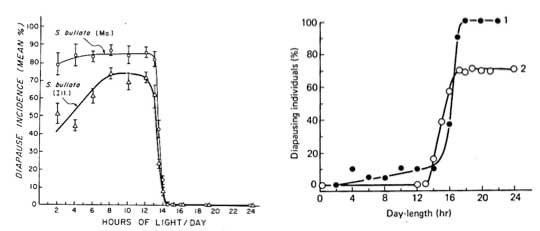
Hierarchy of stimuli in Sarcophagidae (fleshflies)
- Genetic capacity: can select capacity for diapause out of laboratory populations (in bollworms, the ability to diapause is genetically determined).
- Maternal history: mothers that have undergone pupal diapause cannot produce diapausing progeny
- Exposure to short day length as an embryo
- Reinforced by short day length as a larva
- Larva must be exposed to cool temperatures
- Temperature after pupariation must be relatively cool
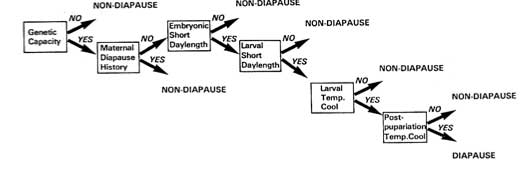
Sequence of events required for the programming of pupal diapause in flesh flies (Sarcophaga).
Endocrine Control of Diapause
There is often a preparatory period before diapause in which the insects’ metabolism is altered. Reserves are directed away from reproduction and development and into storage in the fat body. At the onset of diapause metabolism drops markedly and development halts or slows drastically.
In almost all cases examined, this is controlled by hormones, often due to a reduction in titre of a single hormone that is required for normal metabolism and development.

Role of the Brain in Initiating Diapause
Most evidence suggests that the brain, rather than the eyes or ocelli, is the light receptor organ for photoperiod in relation to diapause.
Experiments include exposing different parts of the body to light, combined with painting over or destroying the eyes.
Brain implant experiments showed that brains implanted into abdomens convey the capacity for photoreception to the abdomen.
Another series of experiments on Manduca sexta showed that brain-retrocerebral complexes cultured in vivo for several days in different day-lengths before reimplantation into recipients could reprogram the diapause response of the recipients.
Embryonic Diapause
Female silkworm Bombyx mori produces diapause hormone which determines the diapause state of the eggs. The production of the diapause hormone depends on the day length the mother was exposed to as an egg.
Ovary transplants showed that a signal in the recipient could determine whether or not they produce diapause eggs.
- Excision of sub-oesophageal ganglion from either “diapause producers” or “non-diapause producers” eliminates diapause.
- Excision of brain from adult “diapause producers” after eclosion causes a drop in proportion of diapausing eggs.
- Brain excision from adult “non-diapause producers” caused an increase in diapause.
Suggests that brain stimulates SOG to produce a diapausing hormone.
A single pair of large neurosecretory cells in the SOG are the source of the hormone, but there is also evidence that neurons in the brain produce the hormone or its precursors.
Diapause hormone has been found in a number of moth species that show egg diapause. However brain extracts from some non-diapausing species of insects have diapause hormone effects, suggesting that the egg diapausers have seconded a protein that originally had and still has another function.
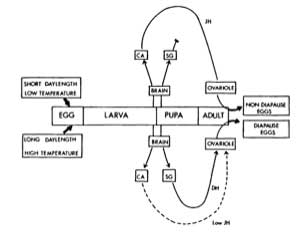
Schematic overview of the environmental and neuroendocrine events leading to the production of diapause and nondiapause eggs by females of the silkmoth Bombyx mori. CA = corpus allatum: SG = subesophageal ganglion DH = diapause hormone: JH = Juvenile hommone.
Larval Diapause
An extreme example is the 4 year developmental arrest of larvae of 17-year cicadas.
In final instar of lepidoptera, diapause is mainly due to inhibition of PTTH and ecdysteroid secretion, preventing the moult to the pupal stage.
In some species PTTH inhibition is due to maintenance of high levels of JH (JH is normally switched off in the middle of the final instar). Demonstrated by applying JH ectopically to non-diapausing larvae.
Suggests a mechanism by which diapause evolved.
Some lepidoptera undergo “stationary” moults in which there is no growth between moults.
Pupal Diapause
Common in Lepidoptera and higher Diptera.
Due to inhibition of PTTH release (and consequent lack of ecdysteroid release).
In Manduca there is further suppression of the prothoracic glands’ ability to release ecdysteroid.
Development can be re-initiated by injection of ecdysone.
Frequently a period of chilling of diapause pupae is required to break diapause.
Adult Diapause
 |
Juvenile hormone titers in diapausing and nondiapausing female adults of Leptinotarsa decemlineata. Animals reared under longday conditions (dashed line) have JH titers that rise soon after adult eclosion (day 0). Animals reared under short days (solid line) enter diapause, and their JH titers fall soon after adult eclosion. When females break diapause their JH titer immediately rises. (Redrawn from De Kort, 1981.) |
Characterised by a cessation of egg development, egg resorption, increase in fat body, alteration of behaviour and physiology. In some insects, the flight muscles degenerate and the proteins and nutrients are recovered. Some undergo marked behaviour changes such as the migration of monarch butterflies.
In most adult insects suppression of JH is the cue for diapause. JH is normally required for development of the gonads.
Genetics
The pink bollworm, Pectinophora gossypiella, diapause as larvae in the soil or in cotton seeds. The diapause trigger is a recessive gene: 2 copies are required for diapause, i.e. the gene must be homozygous. Heterozygotes do not diapause.
The recessive condition for diapause triggering was considered seriously in a genetic control model that started with rearing large numbers of non-diapause bollworms and releasing them into cotton-growing areas in autumn. The method would rely on the non-diapause insects mating with wild populations to produce heterozygotes that then would not survive the winter.
References
Denlinger, In: Comprehensive insect physiology, biochemistry and pharmacology / executive editors: G.A. Kerkut, L.I. Gilbert. Pergamon Press, 1985. Vol 8. Chapter 11. QL495.C64
Nijhout, H.F. (1994) Insect Hormones. Chapter 7.
TOPIC REVIEWDo you know…?
|