Moulting
Objectives
- Know the major hormones involved in moulting.
- Know how the different hormones are regulated to act in concert
- Appreciate the difference between hormonal regulation of moulting in hemimetabolous and holometabolous insects
- Know the role of eclosion hormone and eclosion triggering hormone in moulting.
Topic Outline
- Part 1: Hormonal control of moulting
- Part 2: Ecdysone
- Part 3: Juvenile H
- Part 4: Eclosion and Ecdysis triggering hormone
Activities
- Listen to the minilecture on Eclosion and Ecdysis Triggering Hormone (in Part 4)
Part 1: Hormonal control of moulting
History of PTTH (brain hormone) and ecdysone (moulting hormone)
1910s and 1920s: Ligature experiments by Kopec
If a gypsy moth (Lepidoptera) final instar larva is ligated and bisected after the final larval moult, the front half pupates while the posterior half does not, though it remains viable for weeks. Kopec concluded that the brain is the source of a substance required for metamorphosis.
1930s: endocrine control of moulting
Wigglesworth carried out many experiments on the endocrine control of moulting in the 1930s. His chosen insect was Rhodnius prolixus, a blood-sucking reduviid that transmits Chagas’ disease (a trypanosomiasis) in Central and South America. The larvae require a large blood meal before a moult can occur.
|
In Rhodnius, head removal resulted in a long-lived larva that did not moult. If removal of brain is delayed past a critical phase after the blood meal, the moult ensues anyway. |
|
The “head critical period” was defined as the time at which the moult can proceed independent of the brain, as determined by ligature or removal (approximately 3 days in Rhodnius). |
||
Parabiosis of Rhodnius larvae showed that an activated larva (i.e. some days after feeding and past the head critical period), when parabiosed to a brain-removed larva could induce moulting in the brain-removed larva, therefore an endocrine factor was responsible because no neural or epidermal connectivity existed between the parabiosed larvae. |
||
Implantations of parts of CNS allowed the hormone source to be localised to the anterior part of the brain: |
||
--Suboesophageal ganglion: no effect, |
||
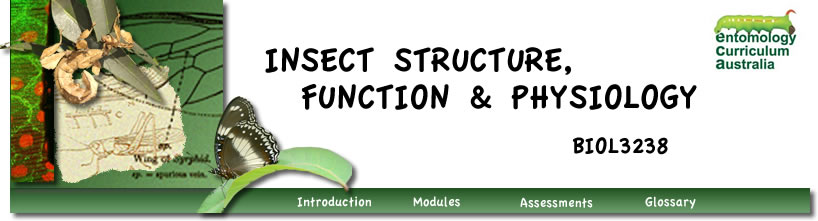
Histograms of head critical periods for the five larval instars of Rhodnius prolixus. Bar height indicates percentage of larvae which molted when decapitated at various times atter a blood meal. (Redrawn from Wigglesworth 1934.)
1947-48: Williams working on the silkmoth
An "activated" brain placed in anterior half of bisected pupa caused moult, while activated brain placed in posterior did not cause it to no moult.
The posterior half moults only when activated brain and prothoracic gland implanted.
He proved that the “brain hormone” acted upon the prothoracic gland to initiate a moult. Subsequently this "brain" hormone was renamed prothoracicotropic hormone (PTTH).
Secretion of PTTH from brain
PTTH is a neurosecretory polypeptide, produced by neurosecretory cells located in the brain. PTTH regulates the secretion of ecdysteroids by the prothoracic glands.
In most insects, the neurosecretory axons project to the corpora cardiaca. However the exception is the lepidoptera, where the corpora allata are the neurohemal organs.
PTTH is turned on for a short period to initiate the moult. In Rhodnius, PTTH release is required for several days while inManduca it is required for only a few hours. In this insect it acts as a trigger.
What is the moulting stimulus?
In Rhodnius, stretch receptors in the abdomen monitor abdominal distension from blood meal
Other hemiptera use the same process: Oncopeltus injected with saline will moult to a small adult.
In Lepidoptera the exact stimulus is unknown. It is known that: Manduca larvae stretch receptors input is not the important cue. In most Lepidoptera the stimulus is closely correlated with the insects’ size, but exactly how this is monitored and measured and triggers PTTH release is unknown.
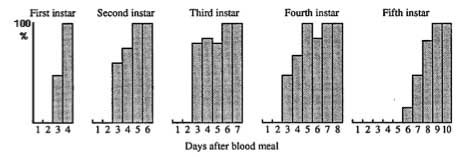
 |
Brain-retrocerebral complex of a fifth instar larva of Manduca sexta. Immunohistochemical staining with an antibody to PTTH. The lateral neurosecretory cells of group III (LNSC III) are the primary sources of this hormone, which is transported via the nervi corporis cardiaci I and II (NCC I+II) and released from the corpus allatum (ca). The corpus cardiacum (CC) is not a neurohaemal organ for PTTH. (From Nijhout, 1994.) |