Moulting
Part 4: Eclosion and ecdysis triggering hormone
Determines the onset of ecdysis: regulates many behavioural and physiological aspects of ecdysis, including a stereotyped series of behaviours which are associated with ecdysis and function to loosen the old cuticle and allow the pharate to extract itself from the exuvium.
In the silkmoth, pupal pre-ecdysis and ecdysis behaviour includes:
- rotatory movements of abdomen: 30 min rotation followed by 30 min quiet (1 hr duration)
- during adult eclosion: peristaltic pumping movements, shrugging of wing bases.
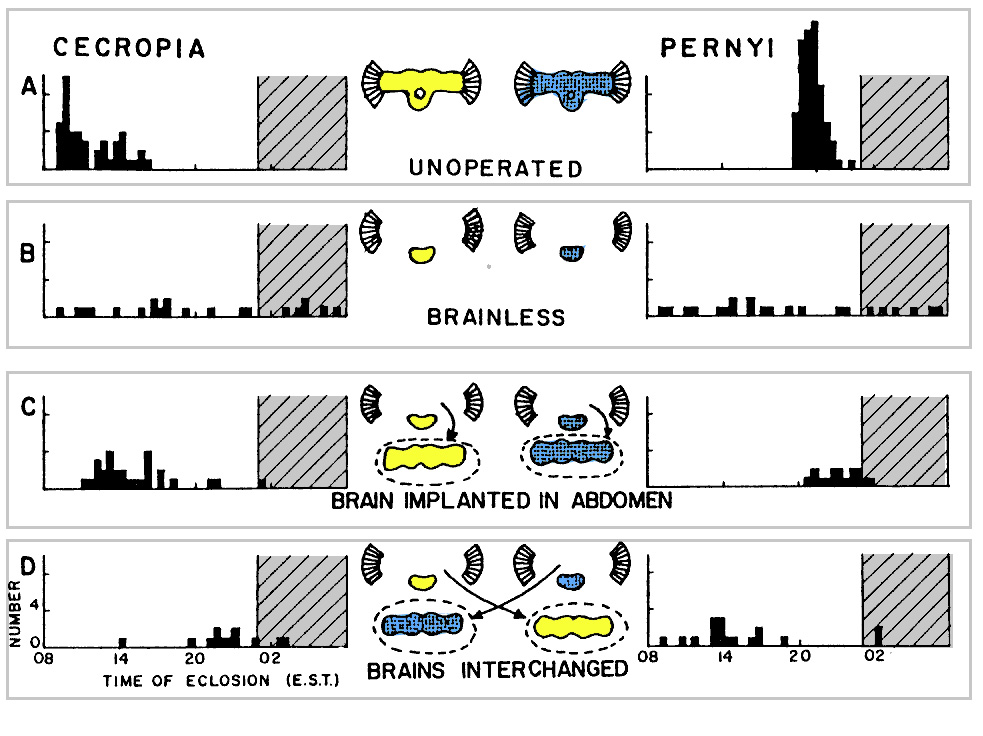
Timing of eclosion of Hyalophora cecropia and Antheraea pernyi under a 17L: 7D photoperiod regimen (daytime is white, nighttime is cross-hatched). (A) Speciesspecific eclosion times of intact unoperated animals. (B) Brainless animals eclose at random times during the day and night. (C) Brains reimplanted into the abdomens of brainless animals restore the normal eclosion time for each species. (D) Brains interchanged hetween the two species and implanted into the abdomen cause each to eclose at the time characteristic of the brain donor, although the eclosion behavior remains characteristic of the recipient (From Truman, 1971b)
Truman and Riddiford investigated the circadian rhythm of eclosion in adult silkmoths. A number of experimental interventions were carried out to discover the source of the circadian periodicity of eclosion:
- Brain removal: eclosion rhythm becomes “free running”
- Brain reimplantation: eclosion rhythm is restored to reflect the day-light regime experienced by the implanted brain
- Cross-species implantation: resulting eclosion rhythm is characteristic of the brain donor species
Conclusions:
- A brain-centered component,
- A preprogrammed ventral ganglia-associated component
- A circadian clock restricts EH release to a certain time of day which gates the time at which adult eclosion occurs.
|
Source
Eclosion hormone is a neuropeptide released from VM cells in brain (2 neurons per side). The neuropeptide has 62 amino acids. 80% similarity between Manduca sexta and Bombyx mori.
The neurosecretory neurons have very long axons that run through the thoracic and abdominal ganglia and out the proctodeal nerves to a neurohemal area along the proctodeal nerves. Stimulation of these neurons causes release of EH. The neurons accumulate secretion between moults and release it in a single, massive bout, as demonstrated by immunostaining and electron microscopy.

EH was originally thought to be involved in adult ecdysis (eclosion) only—hence its name—but found to be involved in ecdysis at all moults (Truman et al., Nature 291:70-71 1981).
Larval moults are determined by developmental cues so that the timing of EH release is determined by the time of ecdysone release. EH release occurs in response to the dropping phase of the ecdysteroid titre, best seen in the larva-pupa transition which is delayed by injection of ecdysteroid. A dose-dependent delay in the time of subsequent ecdysis occurs. In the case of adult ecdysis the delay is not entirely dose-dependent, rather it is gated according to the day-night regime, i.e. it is over-ridden by a circadian rhythm.
There are 2 release sites: within the CNS, and the proctodeal neurohaemal area. Experimental manipulations of Manduca have shown that neuropeptide release in the CNS in the thoracic and abdominal ganglia is responsible for eclosion behaviour. The peptide released from the neurohemal area has an influence on Verson's glands and perhaps other sites such as muscles.
Isolated abdomens show aspects of the eclosion behaviour when injected with EH and isolated nervous system showed associated electrical activity (but only when trachea present) Truman '78 JEB 74:151. The CNS has inherent pattern generators or a neural “program” that is switched on by EH.

Ecdysis Triggering Hormone
Discovered 1996: until then, it was thought that EH was solely responsible for eclosion behaviour. Manduca sexta ETH was shown to initiate pre-eclosion and eclosion behaviours upon injection of extracts with a shorter latency than with injection of EH.
Mas-ETH is a 23 amino acid polypeptide.
The hormone originates in large peripheral neurons or secretory cells associated with the trachea near the spiracles (called Inka cells). The presence of these glandular structures was known beforehand but their function was unknown.
Zitnan, et al, showed that ETH added to an isolated nervous system could induce pre-eclosion motor patterns, unlike Truman’s experiments with EH which required the presence of trachea (ostensibly to keep the CNS aerated, but in hindsight it is likely that the tracheal supply included the epitracheal glands).
They suggested that EH stimulates the release of ETH which is then acts on the CNS to produce pre-eclosion behaviour.
Hesterlee and Morton (1996) noted that centrally released EH in the absence of ETH is sufficient to release the behaviour. Also, ETH elicits ecdysis motor output only when the connection between the brain and ventral nerve cord is intact, suggesting EH is required for eclosion behaviour.
They produced the working hypothesis that there is a positive feedback loop between the VM and Inka cell hormones so that they promote release of EH and ETH and undergo complete release
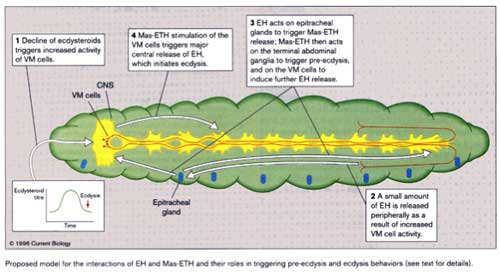
1. Ecdysteroid drop triggers low level VM activity
2. some EH released peripherally (proctodeal neurohaemal region)
3. EH triggers ETH release
4. ETH acts on terminal abdominal ganglion -> preecdysis behaviour
5. ETH acts on VM cells to release more EH
6. Major release of EH triggers ecdysis
In summary, each peptide stimulates the release of the other in a positive feedback cycle.
Recent experiments by Gammie and Truman (1997) show that another neurosecretory product is involved in eclosion. Crustacean cardioactive peptide (CCAP) is produced in a few neurons of each ventral ganglion. EH triggers CCAP release which terminates pre-eclosion motor patterns and initiates eclosion motor patterns.

 Minilecture:
Minilecture: