Reproduction & Oviposition
Objectives
At the end of this module you will know:
- the components of the reproductive system in male and female insects
- about oogenesis, mating and oviposition
Outline
- Male reproductive system
- Female reproductive system
- Mating
- Oviposition
Activities
- Minilecture on reproduction for this module
|
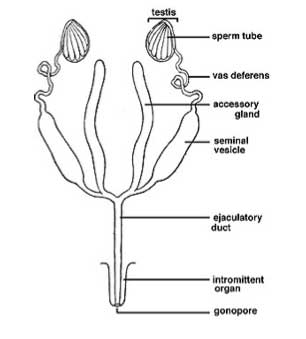
Male Reproductive System
Testes -> vas deferens -> median ejaculatory duct
- The paired testes are bilateral, sometimes fused at the midline.
- Usually composed of many follicles.
- There is a progressive maturation of sperm from distal to proximal in the follicle.
- There is a germarium at the distal end which appears to have a trophic (nutritive) function. Spermatogonia form packets or cysts of synchronously developing cells.
- Three zones of development:
- Zone of Growth: the primary spermatogonia divide via mitotic divisions and increase in size to form spermatocytes.
- Zone of Maturation and Reduction: spermatocytes divide twice (meiotically) to form spermatids
- Zone of Transformation: spermiogenesis occurs (spermatids mature into spermatozoa)
- Spermatozoa are usually filamentous. 300µm to over 1 mm in length. Head and tail are approximately same diameter, unlike most mammalian sperm. Sperm head is occupied by the nucleus, acrosome and mitochondrial derivative.
- Sperm capacitation: changes in the sperm associated with deposition in the female
- The vas deferens usually paired. May be dilated to form the seminal vesicle—a storage organ for spermatozoa.
- The ejaculatory duct is ectodermal and cuticle-lined. Often muscularised due to its function as a sperm pump.
- Accessory glands of various types are present. They may open into the vas deferens or the ejaculatory duct.
- Ectadenia open into the ejaculatory duct, those opening into the vas deferens are mesodermal and called mesadenia.
- Some functions of the accessory gland secretions include:
- activation of the spermatozoa
- spermatophore formation
- release of a mating inhibition factor
- accelerate oocyte formation
- provide nutrition for female.
- Small peptides are likely to be the active factors that affect female via her nervous or muscular system, for example, mating inhibition factor, oviposition-stimulating factor.
Generalised male reproductive system
Female Reproductive System
Ovariole-> lateral oviduct -> common oviduct -> vagina (bursa copulatrix)
- Ovaries are paired, located in the posterior abdomen, can occupy a large volume of the abdomen in gravid (ready to oviposit) females.
- In most insects there are many ovarioles associated with each ovary. However there is variation between species. In Glossina (tsetse) there are 2 ovariole per ovary, in Drosophila 16 per ovary, and in termite queens, over 2000 per ovary. Large number of trachea associated with ovaries to accommodate their high energy demands.
- A duct, termed a pedicel, present at the base of each ovariole.
- The common oviduct is muscle lined. Frequently it has a muscular expansion called the bursa copulatrix. Can have a role in mating, receiving the aedeagus and secretions from the male (spermatophore, spermatozoa, accessory gland secretions), and/or a role in oviposition, as it is the site of egg fertilisation and coating with female accessory gland secretions.
- There are generally one or more pairs of accessory glands opening into the bursa copulatrix or common oviduct. Often they secrete an adhesive which coats the eggs to stick them together or to the substrate. Glands with this function are called colleterial glands. Some form an ootheca (cockroaches, mantids). In tsetse flies they form milk glands producing a nutritive secretion for the larva that is developing in the uterus.
- Spermathecae are cuticle-lined reservoirs where sperm are stored after mating. The spermathecal ducts are often very narrow, and the spermathecae bulbous. Spermathecae are often surrounded by glandular cells that probably provide nutrient to the spermatozoa, though they may play a role in aiding spermathecal filling.
- Terminal filament (Muller's Thread, terminal ligament)
- germarium - region of division and differentiation
- contains germ cells (oogonia) responsible for producing oocytes. Oogonia are divided into:
- primary oocytes
- trophocytes (nurse cells).
- also contains follicle cells which:
- accumulate around oocyte as they move along the germarium
- manufacture or deposit the chorion of the egg shell
- have a role in yolk formation.
- remain within the ovariole either during ovulation or passage of the egg from the lateral to median oviduct.
Vitellarium - zone of growth
- large part of the ovariole in which oocytes grow by deposition of yolk in a process called vitellogenesis
- enlarges rapidly as the oocytes multiply and mature
- ovaries in length depending on the number and size of eggs
Pedicel - duct joining the ovariole and the lateral oviduct
- a narrow duct or constriction at the base of the ovariole.
- a plug forms between the vitellarium and the pedicel in immature insects and between ovipositional episodes of female insects.
- pedicels fuse to form the calyx which unites the ovarioles with the lateral oviduct
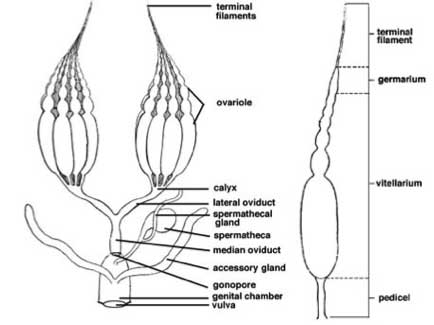
Generalised female reproductive system, Snodgrass 1993
Oogenesis
- The germarium at the distal tip of the ovariole contains the female germ cells (oogonia).
- Oogonia undergo a mitotic division to become primary oocytes.
- Oocytes pass down the ovariole wrapped by follicle cells. They form a series of bulges down the length of the ovariole. Each bulge is called a follicle.
- Vitellogenesis is the process by which oocytes incorporate yolk.
Three types of ovarioles:
- Panoistic: no trophocytes, seen in more primitive insects.
- Telotrophic: the trophocytes in the distal end of the ovariole remain in contact with the oocyte through long tubes (nutritive cords) that carry nutrient into the oocyte. Found in Hemiptera and some Coleoptera
- Polytrophic: Trophocytes (nurse cells) are intimately associated with each oocyte and enclosed within the follicle. Typically, they arise from incomplete divisions of a single oogonium in which the cell divides but doesn’t completely separate. Found in Lepidoptera, Neuroptera, Diptera, Hymenoptera, some Coleoptera.
Follicle cells secrete the chorion (egg shell) late in vitellogenesis.
Vitellogenesis occurs in the proximal part of the ovariole, termed the vitellarium. Nutritive cords are broken about the time of vitellogenesis in telotrophic ovarioles. Massive increase in volume.
In polytrophic ovarioles, the follicle epithelium pulls away from the oocyte and gaps appear between them. Vitellogenin is incorporated from the haemolymph into the oocyte by receptor-mediated endocytosis.
Lipid yolk and protein yolk are derived from the fat body and circulated via the haemolymph.
Hormonal controls
Juvenile hormone from the corpora allata forms a peak in the adult. Necessary for vitellogenin release from fat body.
In some insects the oocytes develop in the pupa.
In others, certain nutrients are required:
autogenous insects: don’t require a protein meal as an adult for oogenesis to occur
anautogenous insects: require a protein meal for oogenesis.
Oocyte Resorption
Usually a response to adverse conditions. Protein and lipid disappear from the oocyte, resorbed into haemolymph.
Females show a range of strategies:
Some produce eggs asynchronously so that they are always ready to oviposit but in small numbers. Common in lepidoptera.
Some produce synchronously and lay in batches of several hundred eggs. In some cases, it has been shown that accumulations of larvae are an advantage, especially when food is not a limiting resource.
For example, blowflies produce batches of eggs and larvae are often more likely to survive in numbers. They even produce an oviposition pheromone.
Fertilisation
Ovulation is the process of release of an oocyte from the ovariole. Usually it is immediately followed by fertilisation and oviposition, but not necessarily. Some insects store eggs in the lateral oviducts after ovulation and fertilise and oviposit in rapid sequence.
Ovulation is induced by neurosecretory factor from the brain.
Fertilisation usually occurs in the bursa copulatrix or similar structure in the common oviduct. Eggs are held momentarily near the opening of the spermathecal ducts. Sperm are released and enter the egg at the micropyles.
In Lucilia and other flies the ovipositor is extruded, the common oviduct has an “S”-shaped bend. The eggs lodge one at a time in the S where they are opposite the spermathecal duct opening and there is a felt-work of fine cuticular spines. They are fertilised and deposited.
Mating
Pheromones are important in mating, especially in mate finding, but also in mate acceptance at close range (more about this in the next module: semiochemicals).
Female receptivity appears to be closely related to JH levels in many insects. This links with presence of mature oocytes.
Female receptivity is frequently terminated after mating, attributable to:
- the presence of sperm
- the mechanical stimulation associated with mating
- a receptivity-inhibiting substance (a pheromone) in the male accessory gland material.
Males produce many spermatozoa.
It is to their advantage to inseminate as many females as possible and to prevent females from subsequently remating.
Several strategies:
- inject receptivity inhibiting substance
- mate guarding
- induce female to oviposit immediately
Some insects transfer sperm in a spermatophore, others use direct sperm transfer.
Haemocoelic insemination is a bizarre form of insemination found in some bugs. The male ruptures the female reproductive tract and sperm is deposited into the haemolymph where it migrates to special pouches (conceptacula seminales) near the oviducts. Sperm fertilise eggs in the ovary. An evolutionary trend is recognisable. Females have developed special sperm-receiving structures at the point where the male breaks the cuticle. It has been suggested that this provides some nutrient to the females (bugs are parasitic). |
Oviposition
A wide range of oviposition strategies are seen in insects, from almost random casting of the eggs, to highly selective site location. Eggs may be deposited singly or in batches. Generally, there is an important sensory component, either chemical (odours, tastes, stimulatory or deterrent plant compounds, etc) or physical (crevices, formation of regular batches, insertion of eggs into specific regions, boring into plants, etc).
Oviposition and mating in Lucilia species
5 phases:
1. Approach to attractants
2. Searching and settling
3. Preparation for egg laying
4. The act of oviposition
5. Post oviposition stage.
Females are:
- anautogenous
- undergo synchronous ovarian development
- lay eggs in batches of 250 or so
If inadequate protein is available females resorb oocytes in the early vitellogenic stage.
Males reach sexual maturity without ingesting protein but protein fed males are more sexually active. Females become receptive to males only after they have ingested protein. Receptivity increased more rapidly in females given protein at day 6. Interestingly, the amount of protein required for full receptivity is less than that required for oocyte maturation (Barton Browne et al, 1976). It is likely that protein feeding causes release of juvenile hormone which acts upon the ovaries and also initiates the onset of receptivity.
Sperm transfer and utilisation
- On average approximately 3000 sperm transferred at mating.
- 38% of the stored sperm is used at each oviposition event (egg batch deposition). This is interesting because each egg batch is approximately the same size, yet the females use a constant proportion of the stored sperm for each egg batch.
- Indicates that the efficiency of sperm use increases as the sperm store diminishes—15 sperm per egg when 10,000 sperm in store, to 1.5 sperm per egg when 1000 in store.
- However, when the sperm stores drop below about 600 the fertility of the batch reduces, suggesting there is an optimal number of sperm required in the spermathecae for successful fertilisation of each egg in a batch.
- Males can mate multiple times. Experiments established that after 8 or so sequential copulations the number of sperm transferred begins to decrease and the fertility of egg batch laid by the mated female starts to drop.
The switch-off and return of female receptivity
There is a gradual return to receptivity. 24 hrs after mating less than 2% of females are receptive, however after 16 days, 24% are receptive. If given an opportunity to lay eggs, receptivity returns more quickly (33% after 16 days).
It is unlikely that the presence of sperm determines whether a female will remate. Females kept without opportunity to lay will gradually return to receptivity, but their sperm store remains intact.
Injection of one male equivalent of accessory gland material into gravid or virgin females switches off receptivity.
It also causes them to lay (of course, the eggs are infertile).
If the males have been serially mated, the ability to switch off receptivity decreases as the accessory glands are depleted.
Castrated males (testes removed, accessory glands intact) show a reduced ability to decrease receptivity in females: this was unexpected, but explained by the fact that a considerable proportion of the accessory gland material went into the spermathecae where it may be ineffective.
Accessory Gland Material switches off receptivity and switches on the propensity to oviposit: is the same compound responsible?
References
General
Raabe, M (1986) Insect reproduction: regulation of successive steps. Advances in Insect Physiology. 19: 29-154.
Chapman: Chapters 25, 34
Mating in Lucilia
Barton Browne, L., Bartell, L.J., Van Gerwen, A.C.M. and Lawrence, L.A. (1976). Relationship between protein ingestion and sexual receptivity in females of the Australian sheep blowfly Lucilia cuprina. Physiological Entomology 1, 235-240.
Barton Browne, L., Smith, P.H., van Gerwen, A.C.M. and Gillott, C. (1990). Quantitative aspects of the effect of mating on readiness to lay in the Australian sheep blowfly, Lucilia cuprina. Journal of Insect Behavior 3, 637-645.
Barton Browne, L., van Gerwen, A.C.M. and Smith, P.H. (1987). Relationship between mated status of females and their stage of ovarian development in field populations of the Australian sheep blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Bulletin of Entomological Research 77, 609-615.
Merritt, D.J. (1989). The morphology of the phallosome and accessory gland material transfer during copulation in the blowfly, Lucilia cuprina (Insecta, Diptera). Zoomorphology 108, 359-366.
Smith, P.H., Barton Browne, L. and van Gerwen, A., C,M. (1989). Causes and correlates of loss and recovery of sexual receptivity in Lucilia cuprina females after their first mating,. Journal of Insect Behaviour 2, 325-337.
Smith, P.H., Barton Browne, L. and van Gerwen, A.C.M. (1988). Sperm storage and utilisation and egg fertility in the sheep blowfly, Lucilia cuprina. Journal of Insect Physiology 34, 125-129.
Smith, P.H., Gillott, C., Barton Browne, L. and van Gerwen, A.C.M. (1990). The mating-induced refractoriness of Lucilia cuprina females: manipulating the male contribution. Physiological Entomology 15, 469-481.
TOPIC REVIEWDo you know…?
|

 Mini-lecture:
Mini-lecture: