Semiochemicals
Overview
- Semiochemicals are chemicals used as signals: they alter the behaviour or physiology of the receiver.
- Semiochemicals encompass a significant part of the spectrum of sensory modalities that can be received by insects: they exclude tactile and visual signals and include all chemosensory signals that originate from living organisms, including olfaction, taste and internal receptors.
- Semiochemicals do not encompass chemical signals from abiotic aspects of the environment.
- Feeding and mating are the major considerations.
- Semiochemicals can be intraspecific or interspecific.
- Minilectures on semiochemicals
- Selective (no effect on beneficials)
- Non-toxic (delayed onset of resistance)
- Short-lived and non-polluting
- in quarantine control for presence/absence of pests
- When to spray or use other controls, i.e. population density
- Sex attractants not very useful as they only attract one sex, usually males in Lepidoptera
- Aggregation pheromones: Useful in control of Spruce bark beetle in USA
- Potential "lure and kill" for mosquitos: synthetic oviposition pheromone combined with growth regulator insecticide. Affects females which are the blood-feeding sex.
- Sensory fatigue
- Competition between natural and synthetic sources
- Camouflage of natural plumes
- Short life span
- Synchronised mating period
- Short mating period
- Migration of mated individuals into treated area (growers’ groups and area-wide efforts are an advantage)
- Fecundity: in species producing many eggs, the small proportion that manage to mate may still produce sufficient progeny to cause pest problems Would be better if the males attracted the females, then females could be trapped, removing them from the population. Otherwise escaping males can inseminate multiple females. This is almost the rule in Lepidoptera and many other insects
Pheromones |
Intraspecific signalling |
|
Kairomones |
Interspecific signalling |
the responder benefits |
Allomones |
Interspecific signalling |
the sender benefits |
Interspecific chemicals can be signals that originate from a plant and affect an animal, or they can originate from one animal or plant species and affect another.
Interspecific signals are divided into two categories: allomones and kairomones.
The obvious existence of coevolution in the use and purpose of signals makes them an area of interest in evolutionary studies, ecology, sexual selection and pest control.
Activities
|
Pheromones elicit a reaction from members of the sender's species.
Examples |
odour: sex pheromones of moths attract mates |
taste: in many flies, sex pheromones are distributed in the waxes of the cuticle and are detected by taste on contact |
Allelochemicals elicit a reaction from another species
Kairomones: elicit a behavioural or physiological reaction that is favourable to the receiver. For example, exploiters are attracted to the chemicals.
Examples |
plant->insect: sinigrin attracts Pieris (cabbage white) butterflies. |
vertebrate->insect: lactic acid is an attractant for mosquitoes |
|
insect->insect: predators or parasites home in on odours from the host |
Allomones: elicit a behavioural or physiological reaction that is favourable to the sender.
For example, repellent odours, tastes, or toxins. Any chemical that repels predators or parasites is an allomone. Prey immobilisation.
Examples |
insect -> insect or vertebrate: stink bugs release a repugnatorial substance. |
bombardier beetles |
|
bee and wasp stings |
Pheromones
The Chemicals
Includes both volatiles and non-volatiles - many originating from host plants.
In Diptera, cuticular lipids constitute the main sex pheromones. They have low volatility and are mainly detected by contact or close-proximity olfaction. Established that they are released from abdominal oenocytes, but also potentially released from other epidermal cells.
The Receptors
Sensilla, moth antennae, gustatory and olfactory sensilla
Sex pheromones
mate detection: long distance, low concentrations
aphrodisiac
Gland Location
Female lepidoptera, frequently in the terminal abdominal region, often in the soft intersegmental membrane between terminal abdominal segments. Facilitate controlled release.
Calling is elicited by either:
(a) eversion of cuticle
(b) depressing tip of abdomen to reveal intersegmental membrane
Male lepidoptera have hair pencils. Glands open out at base of hairs. Hair pencils everted during calling.
Danaid males can release pheromone-carrying particles from gland-containing pockets on the wings.
Others have special scales (androconia), often on wings that are released and dispersed.
Androconia specialised to carry scent: large surface area at the tips
oviposition pheromone: Lucilia
Anti-oviposition pheromones: frequently seen in parasitoids: avoidance of superparasitism
Pheromonotropic Hormones
PBANS in Heliothis
In Heliothis zea, a neurosecretory product of the brain-suboesophageal ganglion complex is required for pheromone production and release. Demonstrated initially by ligation and transplant experiments. Subsequently a peptide was isolated and the secreting neurons detected by immunostaining.
Pheromone biosynthesis activating neuropeptides (PBANs) produced in neurosecretory cells of the suboesophageal ganglion and stored in the corpora cardiaca (the CC is a neurohemal organ for PBAN). Composed of 33-34 amino acid polypeptides.
Released into haemolymph under circadian control and act directly on the pheromone glands, stimulating pheromone synthesis in the abdominal glands of females.
It is only released in the scotophase (dark), correlating with calling activity of females.
PBAN may also act on the terminal abdominal ganglion to activate nervous stimulation of the glands and increase synthesis and release. Artificial stimulation of the nerves to the pheromone gland cause immediate increase in synthesis of pheromone, even outside of “normal calling hours”.
Appears to be comprised both a nervous and neuroendocrine pathway but this is still controversial.
The peptide is conserved across species. Injection of the PBAN (or SOG homogenate) from one species (or even different orders) into another will produce pheromone release, even though the pheromonal compositions themselves are quite different. Bombyx mori PBAN is approx 80% homologous with the Heliothis PBAN.
Alarm Pheromones
Blum in Kerkut and Gilbert uses the term “glandular parsimony”: insects frequently utilise glands whose original function was quite different.
Mandibular glands in bees and ants.
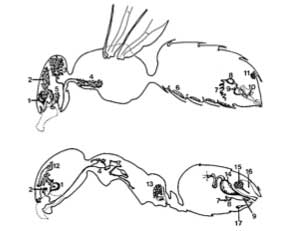
Above. Exocrine glands of the honeybee worker, Apis mellilera (top), and the Argentine ant, Iridomyrmex humilis (bottom). (1) Mandibular glands; (2) hypopharyngeal glands; (3) head labial glands; (4) thoracic labial glands; (5) hypostomal gland; (6) wax glands; (7) poison gland; (8) vesicle of poison gland; (9) Dufour's gland; (10) Koschevnikov's gland; (11) Nasanov gland; (12) postpharyngeal gland; (13) metapleural gland; (14) hindgut; (15) anal gland; (16) reservoir of anal gland; (17) Pavan's gland. The apparent diversify of exocrine glands in the social insects illustrates the importance of chemical communication. From Holldobler and Wilson.
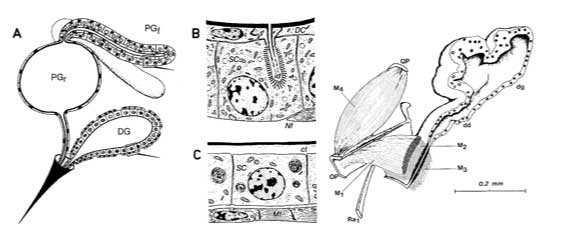
Above. The two major sting glands of Myrmica rubra. (A) General morphology showing the poison gland with its reservoir (PG,) and glandular filaments (PGf), and the Dufour's gland (DG). Small portions of the glandular epithelia of the poison gland (B) and the Dufour's gland (C) are enlarged to show the cytological organization of the secretory cells. SC, secretory cells; id, intracellular ductule; DC, duct cell; Nf, nerve fiber; ct, cuticle; Mf, muscle fiber. Right. Microreconstruction of the Dufour's gland (da) and the adjacent elements showing the four muscle sets (M1-4) affecting the gland duct (dd). Only the parts to the left of the medial body axis are represented; muscles surrounding the gland itself are omitted. OP, oblong plate; QP, quadrate plate; Ra, ramus of the first valvifer. (From Holldobler and Wilson.)
Alarm pheromone released upon stinging by bees, comes from the tissues around the sting apparatus.
Termites produce alarm pheromone in the frontal glands.
Trail pheromones are produced from glands on ventral abdomen
caste determination pheromones
Aggregation pheromones
Cuticular Hydrocarbons
Less studied as sources of pheromones. Most pheromones have been studied because of their possible significance to pest control. The volatile pheromones have most promise in this respect. There is evidence of intersexual signalling by fluid exchange during mating. Male accessory glands can produce receptivity inhibiting substances (injected into female during copulation). Other functions of male accessory gland secretion: stimulate oviposition, and accelerate ovarian development. They appear to be primarily small peptides, unlike most volatile and contact pheromones.
Pheromones: Use in pest control
Advantages:
Monitoring
Most extensive use of pheromones to date:
Used for codling moth Cydia pomonella. Sticky traps commonly used: placed in fruit trees at 1-4 per hectare. Attracts males. Problems with migration into trapping area such as orchard from untreated abandoned orchards.
Mass trapping
Aim is to reduce population by attracting and killing (insecticide or sticky trap).
Mating Disruption
Permeate atmosphere with sex attractant.
Mechanisms:
Successfully used in control of:
pink bollworm, oriental fruit moth, grape berry moth, artichoke plume moth
Best when insect has:
Problems:
Repellents
Limited commercial use. Trials to repel beneficials from treated insecticide areas, e.g. bees as pollinators are often killed. Repelled by treating with alarm pheromone
 |
Rates of JH biosynthesis were determined with an in vitro radiochemical assay and were redrawn from Chiang et al. (1991b); data for basal oocyte length were redrawn from Chiang and Schal (1994). Pheromone content of the gland was determined by electroantennograms of gland extracts. as described in Liang and Schedl (1993c). Schematics are based in part on descriptions in Liang and Schal (1993c). Schematics are based in part on descriptions in Liang and Schal (19C3c). Cu, cuticle D duct: DC duct cell: Dp cuticular depression; EC, epidermal cell; M, mitochondria Nu nucleus; O duct orifice. |
Developmental regulation
Pheromone production in adult sex-specific glands is often coordinated with the appropriate reproductive events. It is not surprising that pheromone production is coordinated with maturation of the gonads and pheromone release is coordinated with the appropriate behavioural or physiological processes.
Glands associated with the reproductive tract frequently develop and start to produce secretions along with the maturation of the gonads.
In some cases, ecdysteroids (moulting hormones) produced by the ovary stimulate production of the sex pheromone.
Blatella germanica
Juvenile hormone, produced by the corpora allata, can induce pheromone production. Blatella germanica females have reproductive cycles. Females attract males after they become sexually mature through release of a sex pheromone from the 10th abdominal tergite.
They carry the ootheca for approx. 21 days before releasing it and becoming sexually receptive again.
The sex pheromone-producing glands undergo cycles of gland secretory production followed by regression. JH controls the cycles of egg development, onset of receptivity and also the cycles of pheromone production. This has been demonstrated by CA removal, reimplantation, and ectopic application of JH analog.
Termination of calling is determined by (a) the presence of a spermatophore in the bursa copulatrix and (b) the presence of sperm in the spermathecae. This appears to be a neural signal (an intact ventral nerve cord is required for termination of calling).
It is not known whether JH acts directly on the glands or whether it acts indirectly, perhaps through a PBAN-like mechanism whereby a humoral factor is released. Such instances are rare but the authors comment that this is probably because there have been few studies of cyclical glandular activity in long-lived species that undergo cycles of receptivity.
References
Raabe, M (1986) Insect reproduction: regulation of successive steps. Advances in Insect Physiology. 19: 29-154.
Chapman, Chapters 15, 34
TOPIC REVIEWDo you know…? |

 Mini-lecture:
Mini-lecture: