Sensing taste and odours
Part 6: Function
The process of stimulation of a chemosensory nerve cell involves 1) the entry of the chemical and 2) the interaction of the chemical with receptors on the surface of the nerve cell.
The perireceptor environment
Little is known about how the chemical gets into the sensillum. How it interacts with the lymph inside the sensillum is better known but there are still a lot of questions to be answered. The best model is shown in this diagram. It has been put together mostly from what is known about the pheromone-sensitive sensilla in silk moths.
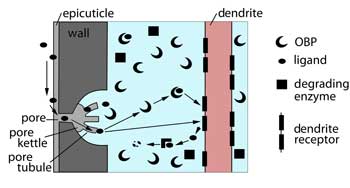 |
A schematic of the process of stimulus transduction external to the sensory neuron. [Diagram: B.W. Cribb] |
The odour molecule (ligand) may enter using a pore tubule. Once inside it can interact with proteins (odour binding proteins: OBPs). These are thought to protect the odour molecule from degredation by enzymes. The odour molecule, with or without the chaperoning protein, links with a receptor on the membrane and this activates the nerve cell. Afterwards the odour molecule is broken down by enzymes in the lymph.
The receptor
Molecular receptors reside in the dendrite membrane. These are molecules that loop back and forth across the membrane and provide on the outer side a region that docks with the chemical (called the ligand). When these are activated by the chemical being smelt or tasted, ion channels open up in the dendrite membrane. The nerve cell undergoes a change. Generally there is a buildup of a receptor potential with this flow of ions through the open channels in the dendrite membrane. When this electrical potential reaches a threshold the cell generates action potentials that travel to the central nervous system down the axon.
There is still a lot to be discovered about the receptors. Table 2 shows the five different types that have been discovered. Within the general olfactory and gustatory receptor class (Ors and Grs) there are a large variety of individual receptors. Depending on the species of insect we look at, there are from 62 -341 Ors and 13 to 88 Grs. But it is not known what they all respond to yet but some have been shown to work together. Generally speaking, each dendrite only contains a few different types of receptors. This means individual sensory cells respond to a limited set of chemicals.
Receptor types
Receptor |
Examples of ligands (from Drosophila) |
Transmembrane morphology (central bar = dendrite membrane. Upper is the outside the dendrite; lower surface is inside the dendrite) |
Olfactory receptors (Ors) |
Many volatile compounds, food odors, cuticle components |
|
Gustatory receptors (Grs) |
Sugars, caffeine, carbon dioxide |
|
Ionotropic receptors (IRs) |
Volatile amines, carboxylic acids, food odours |
|
Transient receptor potential (TRP) channel family |
Humidity and isothiocyanates (as found in wasabi), |
|
Amiloride-sensitive degenerin/epithelial Na+ channels (DEG/ENaC) |
Sensing of salts |
|
? |
Water |
? |
Coding the sensory signal
Insects can recognize not only the presence of a stimulatory chemical in the environment but assess changes in its concentration within space (spatial information). They can also monitor how the chemical concentration shifts across time (temporal information). A moth that is following an odour trail through the air must be able to determine if it is flying towards or away from its mate.
Sometimes insects are better off not responding to a chemical signal. For example, after mating there is little use in responding to the calling chemicals of another mate. After feeding on a host a mosquito needs to digest the meal rather than be drawn to another host. This is achieved by a change in the responsiveness of the sensory nerve cells associated with sensilla.
Early work on mosquitoes showed that feeding affected the lactic acid receptor The electrophysiological activity of the lactic acid-excited neurons was depressed following a blood meal, meaning that mosquitoes would be less likely to respond to a near-by host. The effect was driven by a factor in the hemolymph. (Davis, E.E. (1984) Regulation of sensitivity in the peripheral chemoreceptor systems for host-seeking behaviour by a haemolymph-borne factor in Aedes aegypti. Journal of Insect Physiology, 30, 179-183.).
 |
Aedes aegypti mosquito biting arm. Credit: USDA website at http://www.ars.usda.gov/is/graphics/photos/aug00/k4705-9.htm |
A strong inhibition of host seeking is also seen in Anophelese gambiae following blood-feeding. This lasts 72 hours later (Takken, W., Van Loon, J.J.A. & Adam, W. (2001) Inhibition of host-seeking response and olfactory responsiveness in Anopheles gambiae following blood feeding. Journal of Insect Physiology, 47, 303-310.)
The olfactory system of male Agrotis ipsilon, a noctuid moth, is switched on or off depending on age and reproductive state (Jarriault, D., Gadenne, G., Rospars, J.-P. & Anton, S. (2009) Quantitative analysis of sex-pheromone coding in the antennal lobe of the moth Agrotis ipsilon: a tool to study network plasticity. The Journal of Experimental Biology, 212, 1191-1201.)
Age is important too. Older moths (2-4 days old) of the Egyptian leaf worm Spodoptera littoralis show greater antennal sensitivity than newly emerged moths. (Martel, V., Anderson, P., Hansson, B.S. & Schlyter, F. (2009) Peripheral modulation of olfaction by physiological state in the Egyptian leaf worm Spodoptera littoralis (Lepidoptera: Noctuidae). Journal of Insect Physiology, 55, 793–797.)
Mating reduced antennal sensitivity by 19% in this moth. This reduction may be achieved by activation of biogenic amine receptors linked with the olfactory receptors.
Responsiveness of the peripheral sensory system can be affected by season too and even across a single day.
Drosophila and cockroaches (Leucophaea maderae) show changes in olfactory responsiveness related to time of day and a circadian rhythm is involved (Saifullah A.S.M., & Page, T.L. (2009). Circadian regulation of olfactory receptor neurons in the cockroach antenna. Journal of Biological Rhythms, 24, 144-152.).
The control of feeding
Sugars or specific feeding stimulants will usually start the feeding process and also maintain it. Deterrent substances (bitter, salty or toxic chemicals) will inhibit feeding. But feeding is not a passive event. Insects have been shown to regulate nutrient intake. This involves the peripheral chemosensory neurons (review: Behmer 2009).
When the grasshopper Locusta migratoria and the caterpillar Spodoptera littoralis were restricted to either a protein diet or a carbohydrate diet and then allowed to choose between a diet source high in carbohydrate or in protein they made distinct choices This is because mouthpart chemoreceptors increase in responsiveness to stimulation with amino acids and sugars as titers of these two nutrient groups drop in the hemolymph.
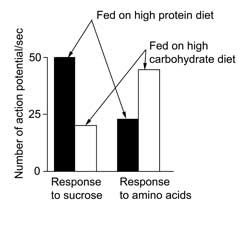 |
Graph of nerve impulses from gustatory sensilla in Locusta. Data from Simpson et al. (1991). Variation in chemosensitivity and the control of dietary selection behaviour in the locust. Appetite, 17, 141–54. |
References related to feeding in locusts:
Abisgold, J.D. & Simpson, S.J. (1988). The effect of dietary protein levels and hemolymph composition on the sensitivity of the maxillary palp chemoreceptors of locusts. Journal of Experimental Biology, 135, 215–29.
Simpson CL, Simpson SJ & Abisgold JD. (1990). An amino acid feedback and the control of locust feeding. Symp. Biol. Hunarica, 39, 39–46.
End of Module: Sensing taste and odours.